YUTREPIA is designed to deliver treprostinil deep into the lungs with minimal inspiratory effort from the patient1
PATIENTS PREFER THE YUTREPIA DELIVERY DEVICE1,§

100% of patients who transitioned from using the TYVASO® nebulizer preferred or strongly preferred the YUTREPIA device when surveyed at 4 months of use.
§Patients in the transition group of INSPIRE (n=49) were surveyed at month 4 on their preference for the YUTREPIA RS00 Model 8 DPI device compared with their previously used device, the nebulized treprostinil inhalation system. Patients were asked if they preferred the old inhalation system, had no preference, preferred the new inhaler, or strongly preferred the new inhaler.1
Watch a video on PRINT® Technology
YUTREPIA LEVERAGES PRINT® TECHNOLOGY TO PRODUCE PARTICLES THAT ARE DESIGNED TO ENHANCE DEEP LUNG DELIVERY1
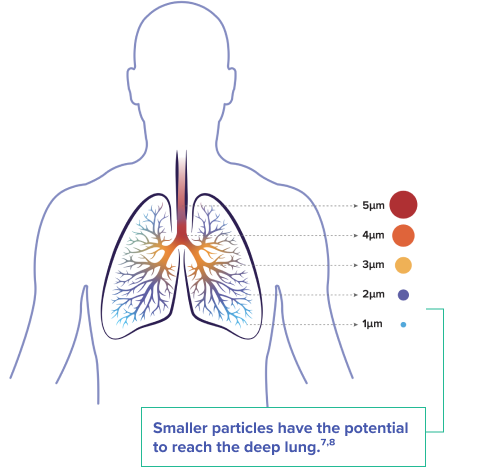

UNIFORM SIZE AND SHAPE1,2,3
- Drug particles <2μm have greater likelihood for lower airway deposition and reduced chance of sedimenting in the upper respiratory tract
- YUTREPIA drug particles are uniform in size (~1 µm) and shape, engineered for enhanced aerosolization and deep-lung deposition of treprostinil
UNIQUE FORMULATION1,4-6
- YUTREPIA's dry-powder formulation aerosolizes into free-flowing particles upon inhalation
- YUTREPIA reduces the need for a patient's inspiratory ability to deaggregate the medication, allowing for use of a low-effort* device
Defined as low inspiratory effort.5,6
YUTREPIA IS DELIVERED VIA A CONVENIENT AND TRUSTED LOW-EFFORT DEVICE1,4-6,9,10,†

LOW-EFFORT
- YUTREPIA's low-resistance device requires minimal inspiratory effort to deliver a dose of treprostinil1,4,‡
- Device is not position-dependent, which may reduce the risk of patient error, spillage, or wasted medication4
CONVENIENT
- Device is a robust, pocket-sized DPI1,4,11
- Both the device and drug capsule are portable and require no refrigeration12
TRUSTED
- The Plastiape RS00 is a low-effort† device that was previously approved by the FDA and EMA4-6,9
- Device has been used for decades across respiratory disease states10
Defined as low inspiratory effort.5,6
In a comparative bioequivalency study of YUTREPIA and TYVASO®, YUTREPIA 79.5 mcg was administered in 1 capsule (2 breaths), which delivered an approximate dose of 56.6 mcg treprostinil. In contrast, TYVASO® was administered in 9 breaths, which delivered an approximate dose of 54 mcg treprostinil. Data are from an open-label study of healthy subjects aged 18-45 years of age. In a crossover sequence, 8 patients received a single dose of each treatment, separated by at least 24 hours.13
DPI = dry-powder inhaler; EMA = European Medicines Agency; FDA = US Food and Drug Administration
INDICATION AND IMPORTANT SAFETY INFORMATION
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
DRUG INTERACTIONS/SPECIFIC POPULATIONS
- The concomitant use of treprostinil with diuretics, antihypertensives, or other vasodilators may increase the risk of symptomatic hypotension.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
- Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality. There are no data on the presence of treprostinil in human milk, the effects on the breastfed infant, or the effects on milk production.
- Safety and effectiveness in pediatric patients have not been established.
- Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open label INSPIRE study in patients with PAH included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
- Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
- No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis.
ADVERSE REACTIONS
- PAH (WHO Group 1): The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
- PH-ILD (WHO Group 3): In a 16-week, placebo-controlled study of 326 patients with PH-ILD (WHO Group 3), adverse reactions with inhaled treprostinil were similar to the experience in studies of PAH.
Please see Full Prescribing Information for YUTREPIA.
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
DRUG INTERACTIONS/SPECIFIC POPULATIONS
- The concomitant use of treprostinil with diuretics, antihypertensives, or other vasodilators may increase the risk of symptomatic hypotension.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
- Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality. There are no data on the presence of treprostinil in human milk, the effects on the breastfed infant, or the effects on milk production.
- Safety and effectiveness in pediatric patients have not been established.
- Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open label INSPIRE study in patients with PAH included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
- Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
- No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis.
ADVERSE REACTIONS
- PAH (WHO Group 1): The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
- PH-ILD (WHO Group 3): In a 16-week, placebo-controlled study of 326 patients with PH-ILD (WHO Group 3), adverse reactions with inhaled treprostinil were similar to the experience in studies of PAH.
Please see Full Prescribing Information for YUTREPIA.
References
- Hill NS, Feldman JP, Sahay S, et al; INSPIRE study investigators. INSPIRE: safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH). Pulm Circ. 2022;12(3):e12119. doi:10.1002/pul2.12119
- Roscigno RF, Farrer BT, Sprague JJ, Maynor B, inventors; Liquidia Technologies, Inc, assignee. Dry powder treprostinil for the treatment of pulmonary hypertension. US patent application 16/099,135. May 5, 2017.
- Chaurasiya B, Zhao YY. Dry powder for pulmonary delivery: a comprehensive review. Pharmaceutics.
- Patel S, Prabel J, MacLennan D, Rosen G. Robustness of YUTREPIA™, a dry-powder inhaled formulation of treprostinil, in patient misuse scenarios. Poster presented at: CHEST 2022 Annual Meeting; October 16-19, 2022; Nashville, TN.
- Price D, Chrystyn H. Concept review of dry powder inhalers: correct interpretation of published data. Multidiscip Respir Med. 2015;10:36. doi:10.1186/s40248-015-0033-0
- National Health Service Sunderland. Sunderland COPD Inhaler Guide. National Health Service; 2020.
- Henao MP, Kraschnewski JL, Bolton MD, Ishmael F, Craig T. Effects of inhaled corticosteroids and particle size on risk of obstructive sleep apnea: a large retrospective cohort study. Int J Environ Res Public Health. 2020;17(19):7287. doi:10.3390/ijerph17197287
- Maynor BW, Anderson S, Vaughn T, et al. Nonclinical, in-silico, and clinical evaluation of LIQ861 inhalation powder deposition and pharmacokinetics. Respir Drug Deliv. 2020;2:371-374.
- Kingman M, Patel, S. Quality of life (QoL) in PAH patients receiving an inhaled dry powder treprostinil (LIQ861) in the INSPIRE study. Poster presented at: PHA International PH Conference and Scientific Sessions; June 9-11, 2022; Atlanta, GA.
- World Pharmaceutical Frontiers. Keep it simple and single. Updated November 5, 2015. Accessed October 9, 2023. https://www.worldpharmaceuticals.net/contractors/drug-delivery-systems/plastiape/
- Hill NS, Feldman JP, Sahay S, et al; INSPIRE study investigators. INSPIRE: safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH). Pulm Circ. 2022;12(3)(suppl):e12119. doi:10.1002/pul2.12119
- YUTREPIA. Prescribing information. Liquidia Technologies, Inc; 2025.
- Roscigno RF, Vaughn T, Hunt T, Parsley E, Eldon M, Rubin LJ. Pharmacokinetic (PK) performance of LIQ861 and evaluation of comparative bioavailability with Tyvaso® in healthy subjects (Study LTI-102). Poster presented at: 14th Pulmonary Vascular Research Institute (PVRI) Annual World Congress on Pulmonary Vascular Disease; January 30-February 2, 2020; Lima, Peru.