Prostacyclin therapy is an established, efficacious treatment for patients with PAH1,2
THE ROLE OF PROSTACYCLIN THERAPY AND ITS CLINICAL BENEFIT FOR PATIENTS1,2
Prostacyclin therapy addresses the underlying pathophysiology of PAH by1,2:
- Inducing vasodilation of pulmonary arteries
- Inhibiting platelet aggregation
- Exerting anti-proliferative and anti-inflammatory effects
Clinical benefits of prostacyclin therapy are well established and include1,2:
- Reduction in symptoms of PAH
- Improved prognostic measures of risk, such as exercise capacity and functional class
- Delayed disease progression
A RETROSPECTIVE ANALYSIS REPORTED LOW PROSTACYCLIN USE AMONG THE 13,633 PATIENTS WITH PAH WHO WERE ANALYZED3
In patients with PAH in the Medicare and Truven Commercial databases (N = 13,633)*
Prostacyclin use across calendar-year cohorts ranged from 19.9% to 22.6%.

Data from a retrospective study of adult patients with PAH who were included in the Truven Commercial and Medicare databases between January 1, 2010, and October 31, 2015. Patients were identified based on claims with ICD-9-CM diagnoses indicative of PAH and claims for PAH-specific medications and PAH-related procedures. Annual cohorts of patients were identified for analysis.
PAH = pulmonary arterial hypertension
PREVIOUSLY EXISTING DELIVERY OPTIONS FOR PROSTACYCLIN THERAPY HAVE LIMITATIONS4,5
These limitations may make it difficult to initiate treatment and titrate to doses that provide full therapeutic benefit4,5
Oral6,7
KEY BENEFIT: CONVENIENT
LIMITATIONS
- Gastrointestinal, nervous, and vascular system side effects
- Requires up-titration, which may be challenging given side effects
Nebulized5,8
KEY BENEFIT: TARGETED
LIMITATIONS
- Burdensome, requiring time and effort
- Frequent administration and multiple breaths are required to achieve a therapeutic dose
- Few patients are prescribed more than 12 breaths QID
DPI9,10
KEY BENEFIT: CONVENIENT
LIMITATIONS
- High resistance/high effort device, placing burden on the patient’s inspiratory ability to deagglomerate the dry powder, which may result in drug particles of various sizes
- Position-dependent device, which may increase the risk of patient error, spillage, or wasted medication
Parenteral6,7,11
KEY BENEFIT: EFFECTIVE
LIMITATIONS
- Potential to cause systemic toxicities and infection
- Can result in site pain and lifestyle limitations
DPI = dry-powder inhaler; IV = intravenous; QID = four times daily; SC = subcutaneous
IN A RETROSPECTIVE CLINICAL ANALYSIS, HIGHER DOSES OF INHALED TREPROSTINIL SOLUTION HAVE BEEN SHOWN TO IMPROVE DISEASE CONTROL5
In patients with PAH in a specialty pharmacy database (N = 5000), those taking >9 breaths QID had5,†,‡:
- Higher survival
rates - Longer time to transition
to parenteral therapy - Greater drug
persistence
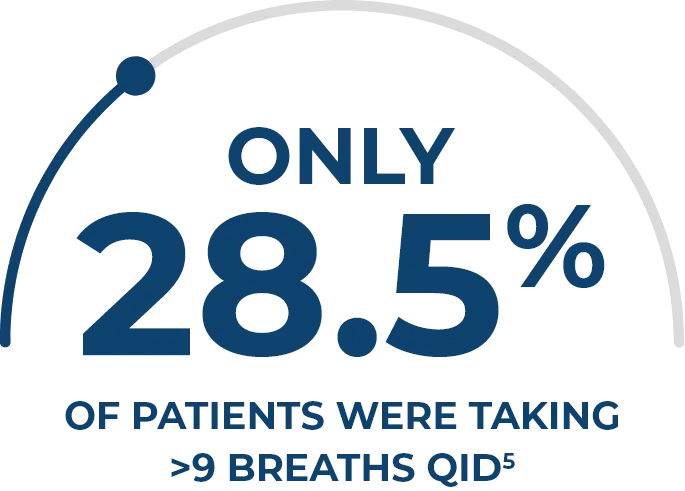
Data from a Phase 4, retrospective, real-world analysis of patients prescribed inhaled treprostinil solution from a specialty pharmacy database between September 2009 and June 2018. Of the 6709 patients who met all study eligibility criteria, a random sample of 5000 patients was selected for further analysis using simple random sampling with equal probability.5
Compared with patients taking ≤9 breaths QID.5
INDICATION AND IMPORTANT SAFETY INFORMATION
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
DRUG INTERACTIONS/SPECIFIC POPULATIONS
- The concomitant use of treprostinil with diuretics, antihypertensives, or other vasodilators may increase the risk of symptomatic hypotension.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
- Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality. There are no data on the presence of treprostinil in human milk, the effects on the breastfed infant, or the effects on milk production.
- Safety and effectiveness in pediatric patients have not been established.
- Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open label INSPIRE study in patients with PAH included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
- Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
- No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis.
ADVERSE REACTIONS
- PAH (WHO Group 1): The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
- PH-ILD (WHO Group 3): In a 16-week, placebo-controlled study of 326 patients with PH-ILD (WHO Group 3), adverse reactions with inhaled treprostinil were similar to the experience in studies of PAH.
Please see Full Prescribing Information for YUTREPIA.
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
DRUG INTERACTIONS/SPECIFIC POPULATIONS
- The concomitant use of treprostinil with diuretics, antihypertensives, or other vasodilators may increase the risk of symptomatic hypotension.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
- Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality. There are no data on the presence of treprostinil in human milk, the effects on the breastfed infant, or the effects on milk production.
- Safety and effectiveness in pediatric patients have not been established.
- Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open label INSPIRE study in patients with PAH included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
- Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
- No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis.
ADVERSE REACTIONS
- PAH (WHO Group 1): The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
- PH-ILD (WHO Group 3): In a 16-week, placebo-controlled study of 326 patients with PH-ILD (WHO Group 3), adverse reactions with inhaled treprostinil were similar to the experience in studies of PAH.
Please see Full Prescribing Information for YUTREPIA.
References
- Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31(4):891-901. doi:10.1183/09031936.00097107
- Mitchell JA, Ahmetaj-Shala B, Kirkby NS, et al. Role of prostacyclin in pulmonary hypertension. Glob Cardiol Sci Pract. 2014;2014(4):382-393. doi:10.5339/gcsp.2014.53
- Burger CD, Pruett JA, Lickert CA, Berger A, Murphy B, Drake W 3rd. Prostacyclin use among patients with pulmonary arterial hypertension in the United States: a retrospective analysis of a large health care claims database. J Manag Care Spec Pharm. 2018;24(3):291-302. doi:10.18553/jmcp.2017.17228
- Hill NS, Feldman JP, Sahay S, et al; INSPIRE study investigators. INSPIRE: safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH). Pulm Circ. 2022;12(3):e12119. doi:10.1002/pul2.12119
- Shapiro S, Mandras S, Restrepo-Jaramillo R, et al. Survival and drug persistence in patients receiving inhaled treprostinil at doses greater than 54 μg (nine breaths) four times daily. Pulm Circ. 2021;11(4):20458940211052228. doi:10.1177/20458940211052228
- Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev. 2015;24(138):630-641. doi:10.1183/16000617.0067-2015
- Coons JC, Miller T, Simon MA, Ishizawar DC, Mathier MA. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients transitioned from parenteral or inhaled prostacyclins: case series and treatment protocol. Pulm Circ. 2016;6(1):132-135. doi:10.1086/685111
- Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care. 2015;60(6):794-805. doi:10.4187/respcare.03927
- TYVASO DPI. Instructions for use. United Therapeutics Corporation; 2022.
- Berkenfeld K, Lamprecht A, McConville JT. Devices for dry powder drug delivery to the lung. AAPS PharmSciTech. 2015;16(3):479-490. doi:10.1208/s12249-015-0317-x
- Burger CD, D'Albini L, Raspa S, Pruett JA. The evolution of prostacyclins in pulmonary arterial hypertension: from classical treatment to modern management. Am J Manag Care. 2016;22(suppl 1):S3-S15.