Suspect, Screen, and Intervene
PH is a common complication in patients with ILD that leads to worse outcomes and increased mortality1


- 3 times greater risk of mortality4
- Lower survival rate at 3 years (~30% for PH-ILD vs ~55% for ILD alone)3,5,6
- Heightened propensity for acute exacerbations4
- Impaired quality of life4
- Lower exercise capacity4
- Greater need for supplemental oxygen4
Data from a retrospective analysis of 170 patients with ILD from 12 international centers. Patients were stratified based on their level of PH according to the following criteria: no PH, mPAP <21 mm Hg or mPAP 21 to 24 mm Hg with a PVR <3 WU; borderline PH, mPAP 21 to 24 mm Hg with a PVR ≥3 WU; mild to moderate PH, mPAP 25 to 35 mm Hg and a CI ≥2.0 L/min/m2; severe PH, mPAP ≥35 mm Hg or mPAP ≥25 mm Hg and a CI <2.0 L/min/m2. Kaplan-Meier analysis with log-rank was used to determine the probability of all-cause mortality based on the level of PH.3
CI = cardiac index; Hg = mercury; ILD = interstitial lung disease; mPAP = mean pulmonary artery pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; WU = wood units
PH IS A COMMON, SERIOUS COMPLICATION ACROSS MANY TYPES OF ILD1
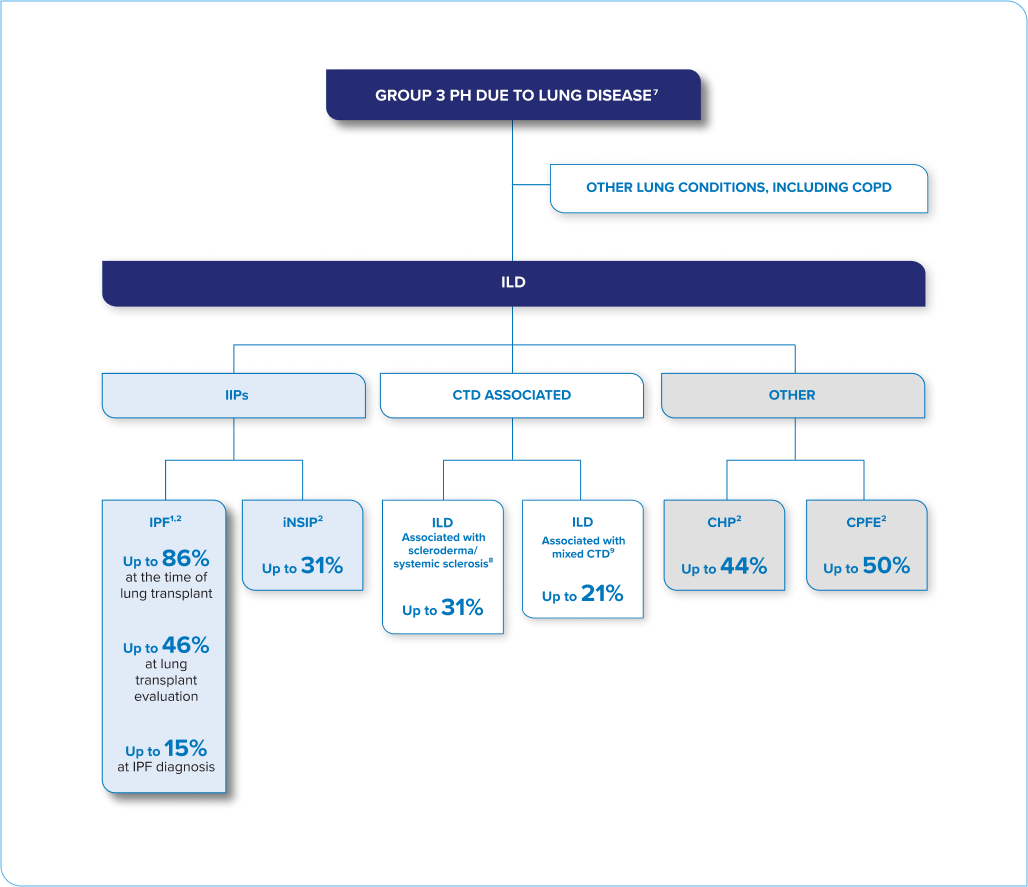
This is not an exhaustive list of ILD conditions.
SCREEN YOUR ILD PATIENTS FOR PH.
PATIENTS WITH PH-ILD MAY BENEFIT
FROM EARLIER INTERVENTION4
CHP = chronic hypersensitivity pneumonitis; COPD = chronic obstructive pulmonary disease; CPFE = combined pulmonary fibrosis and emphysema; CTD = connective tissue disease; IIP = idiopathic interstitial pneumonia; iNSIP = idiopathic non-specific interstitial pneumonia; IPF = idiopathic pulmonary fibrosis
SUSPECT, SCREEN, INTERVENE—EARLY DIAGNOSIS AND INTERVENTION MAY RESULT IN BETTER CLINICAL OUTCOMES4
Three steps to help improve early detection and diagnosis of PH-ILD4
- Monitor for signs and symptoms disproportionate to ILD severity
- Altered heart sounds (loud P2 or S2)
- Jugular venous distention
- Signs of right heart failure
- Ankle swelling/peripheral edema
- Hepatomegaly/ascites
- ILD requiring oxygen
- Perform screening tests
- Pulmonary function tests
- CT scan
- Oxygen saturation
- 6MWD
- BNP/NT-proBNP levels
- Echocardiography
- Confirm PH diagnosis
- Right heart catheterization
Intervene: Inhaled prostacyclin is the only class of therapy FDA approved for PH-ILD10
6MWD = 6-minute walk distance; BNP = brain-type natriuretic peptide; CT = computed tomography; NT-proBNP = N-terminal pro-brain natriuretic peptide; FDA = US Food and Drug Administration; P2 = pulmonic closure sound; S2 = second heart sound
Limitations of previously existing prostacyclin therapy may make it difficult to initiate treatment and titrate to doses that provide full therapeutic benefit11,12
Nebulizer13
- Targeted delivery
- Nebulizers can be burdensome, requiring time and effort
- Frequent administration and multiple breaths are required to achieve a therapeutic dose
Dry-Powder Inhaler (DPI)14,15
- Convenient
- High-effort/high-resistance device, placing burden on the patient's inspiratory ability to deagglomerate the dry powder, which may result in drug particles of various sizes
- Position-dependent, which may increase the risk of patient error, spillage, or wasted medication
INDICATION AND IMPORTANT SAFETY INFORMATION
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
DRUG INTERACTIONS/SPECIFIC POPULATIONS
- The concomitant use of treprostinil with diuretics, antihypertensives, or other vasodilators may increase the risk of symptomatic hypotension.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
- Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality. There are no data on the presence of treprostinil in human milk, the effects on the breastfed infant, or the effects on milk production.
- Safety and effectiveness in pediatric patients have not been established.
- Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open label INSPIRE study in patients with PAH included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
- Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
- No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis.
ADVERSE REACTIONS
- PAH (WHO Group 1): The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
- PH-ILD (WHO Group 3): In a 16-week, placebo-controlled study of 326 patients with PH-ILD (WHO Group 3), adverse reactions with inhaled treprostinil were similar to the experience in studies of PAH.
Please see Full Prescribing Information for YUTREPIA.
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%).
WARNINGS AND PRECAUTIONS
- Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with treprostinil may produce symptomatic hypotension.
- Treprostinil inhibits platelet aggregation and increases the risk of bleeding.
- Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness.
- Like other inhaled prostaglandins, YUTREPIA may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with YUTREPIA.
DRUG INTERACTIONS/SPECIFIC POPULATIONS
- The concomitant use of treprostinil with diuretics, antihypertensives, or other vasodilators may increase the risk of symptomatic hypotension.
- Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8.
- Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, pulmonary arterial hypertension is associated with an increased risk of maternal and fetal mortality. There are no data on the presence of treprostinil in human milk, the effects on the breastfed infant, or the effects on milk production.
- Safety and effectiveness in pediatric patients have not been established.
- Placebo-controlled clinical studies of treprostinil inhalation solution did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. The open label INSPIRE study in patients with PAH included 28 patients aged 65 and over in which no age-related differences were noted. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
- Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency.
- No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis.
ADVERSE REACTIONS
- PAH (WHO Group 1): The safety and tolerability of YUTREPIA was evaluated in an open label study (INSPIRE) of 121 patients with PAH (WHO Group 1 and NYHA Functional Class II [80 patients] and Class III [41 patients]) followed for up to 2 months. The most commonly reported adverse reactions included cough, headache, throat irritation, dizziness, which are known side effects of treprostinil inhalation solution. The adverse reactions in the INSPIRE study were consistent with those observed in previous studies of inhaled treprostinil.
- PH-ILD (WHO Group 3): In a 16-week, placebo-controlled study of 326 patients with PH-ILD (WHO Group 3), adverse reactions with inhaled treprostinil were similar to the experience in studies of PAH.
Please see Full Prescribing Information for YUTREPIA.
References
- Parikh R, Konstantinidis I, O'Sullivan DM, Farber HW. Pulmonary hypertension in patients with interstitial lung disease: a tool for early detection. Pulm Circ. 2022;12(4):e12141. doi:10.1002/pul2.12141
- Nikkho SM, Richter MJ, Shen E, et al. Clinical significance of pulmonary hypertension in interstitial lung disease: a consensus statement from the Pulmonary Vascular Research Institute's innovative drug development initiative-Group 3 pulmonary hypertension. Pulm Circ. 2022;12(3):e12127. doi:10.1002/pul2.12127
- Piccari L, Wort SJ, Meloni F, et al; REHAR Registry Investigators. The effect of borderline pulmonary hypertension on survival in chronic lung disease. Respiration. 2022;101(8):717-727. doi:10.1159/000524263
- Rahaghi FF, Kolaitis NA, Adegunsoye A, et al. Screening strategies for pulmonary hypertension in patients with interstitial lung disease: a multidisciplinary Delphi study. Chest. 2022;162(1):145-155. doi:10.1016/j. chest.2022.02.012
- Chebib N, Mornex JF, Traclet J, et al. Pulmonary hypertension in chronic lung diseases: comparison to other pulmonary hypertension groups. Pulm Circ. 2018;8(2):2045894018775056. doi:10.1177/2045894018775056
- Dawes TJW, McCabe C, Dimopoulos K, et al. Phosphodiesterase 5 inhibitor treatment and survival in interstitial lung disease pulmonary hypertension: a Bayesian retrospective observational cohort study. Respirology. 2023;28(3):262-272. doi:10.1111/resp.14378
- Singh N, Dorfmüller P, Shlobin OA, Ventetuolo CE. Group 3 pulmonary hypertension: from bench to bedside. Circ Res. 2022;130(9):1404-1422. doi:10.1161/CIRCRESAHA.121.319970
- Young A, Vummidi D, Visovatti S, et al. Prevalence, treatment, and outcomes of coexistent pulmonary hypertension and interstitial lung disease in systemic sclerosis. Arthritis Rheumatol. 2019;71(8):1339-1349. doi:10.1002/art.40862
- Hyldgaard C, Bendstrup E, Pedersen AB, Pedersen L, Ellingsen T. Interstitial lung disease in connective tissue diseases: survival patterns in a population-based cohort. J Clin Med. 2021;10(21):4830. doi:10.3390/jcm10214830
- West N, Smoot K, Patzlaff N, Miceli M, Waxman A. Plain language summary of the INCREASE study: inhaled treprostinil (Tyvaso) for the treatment of pulmonary hypertension due to interstitial lung disease. Future Cardiol. 2023;19(5):229-239. doi:10.2217/fca-2022-0108
- Hill NS, Feldman JP, Sahay S, et al; INSPIRE study investigators. INSPIRE: safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH). Pulm Circ. 2022;12(3):e12119. doi:10.1002/pul2.12119
- Shapiro S, Mandras S, Restrepo-Jaramillo R, et al. Survival and drug persistence in patients receiving inhaled treprostinil at doses greater than 54g (nine breaths) four times daily. Pulm Circ. 2021;11(4):20458940211052228. doi:10.1177/20458940211052228
- Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care. 2015;60(6):794-805. doi:10.4187/respcare.03927
- TYVASO DPI. Instructions for use. United Therapeutics Corporation; 2022.
- Berkenfeld K, Lamprecht A, McConville JT. Devices for dry powder drug delivery to the lung. AAPS PharmSciTech. 2015;16(3):479-490. doi:10.1208/s12249-015-0317-x